Interneurons are inhibitory neurons in the brain that are thought to shape the computations performed by principal cells. The effects of inhibition can be rather diverse, depending on which neurons – or which parts of a neuron (dendrites, soma, or the axon hillock) – are targeted. A particularly interesting inhibitory motif that is the disinhibitory motif, which depends on a specific interneuron type known as VIP neuron. VIP neurons primarily inhibit another interneuron type called SST neurons (also written as “‘SOM”), which in turn inhibits principal or pyramidal cells, often by targeting their dendrites. Therefore, activating VIP neurons releases pyramidal neurons from SST-mediated inhibition, possibly creating a window for plasticity and learning.
Unfortunately, both VIP and SST neurons are not homogeneous classes but consist of multiple subtypes which might also vary across cortical and neocortical areas. Below, I’ll highlight a few recent studies on VIP neurons that caught my attention. Let me know if you know others that are similarly interesting!
A role for hippocampal VIP neurons in place cell remapping
In this well-designed and easy-to-read paper, Neubrandt, Lenkey et al. (2025) from the Vervaeke lab investigate how VIP neurons in the hippocampus contribute to place cell remapping. Place cell remapping is a form of hippocampal plasticity where pyramidal cells change their spatial firing fields in response to environmental novelty.
Neubrandt, Lenkey et al. use optogentic tools to activate (ChrimsonR) and inactivate (ArchT) VIP neurons while performing calcium imaging of pyramidal neurons. They find that inactivation decreases remapping and activation increases remapping of pyramidal neurons. The effects are relatively subtle but seem convincing, and align with the idea that VIP neurons facilitate plasticity by disinhibiting pyramidal neurons.
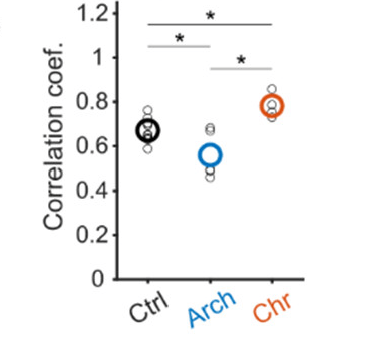
The authors additionally analyze their data with respect to a specific plasticity rule in pyramidal cells termed behavioral timescale synaptic plasticity (covered before on this blog), which results in a more complex picture that is less straightforward to interpret. The authors conclude that in addition to VIP activity other factors (e.g., neuromodulatory input) may be necessary to induce remapping.
Overall, this study nicely shows how novelty activates VIP interneurons to help control plasticity in pyramidal neurons, presumably via SST neurons. Their findings are consistent with and extend previous results from Turi et al. (2019) that demonstrated a necessary role of VIP neurons for reward location learning in hippocampal place cells.
The subtypes of VIP neurons in neocortex
Dellal, Zurita et al. (2025) from Bernardo Rudy’s lab investigate subtypes of VIP neurons in neocortex (somatosensory cortex). They do so in a very systematic study with transgenic animal crossing strategies that enable identification of various subtypes. I really do not want to know how much work it was to cross and validate all these mouse lines! As a result, Dellal, Zurita et al. find four subtypes of VIP neurons.
The most distinctive feature of these subtypes is whether the protein CCK is expressed or not. CCK-negative VIP neurons and in particular CCK-negative CR-positive VIP neurons target mostly SST neurons, while CCK-positive VIP neurons target also pyramidal cells directly and are there not only disinhibitory but also inhibitory for pyramidal cells. It is interesting that very similar findings have been made earlier for hippocampal circuits, where two such subtypes of VIP neurons were found in the 1990s (Acsády et al., 1996; Gulyás et al., 1996).
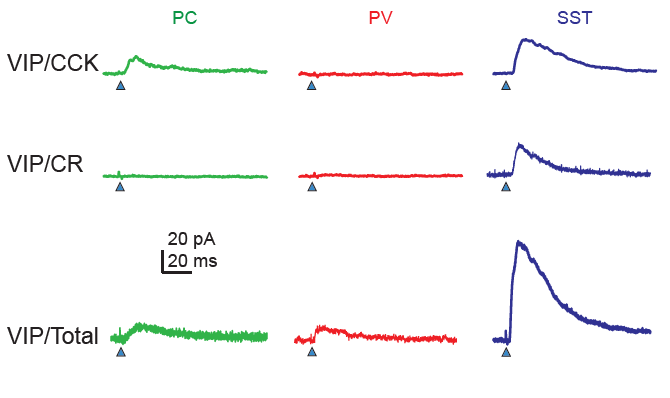
With the increasingly refined functional classification of interneuron subtypes, it becomes more and more difficult to not get confused by the different classification approaches. Other studies (e.g., in Figure 7 by Geiller et al., 2020) used slightly different markers to delineate VIP subclasses, making it quite a puzzle to connect the dots between publications!
A detail that I found particularly interesting as somebody working on the noradrenergic system is the following finding: CR-positive VIP neurons, which target SST neurons but not pyramidal neurons, were not significantly activated by bath-applied noradrenaline (Fig. 6M,N); this is suprising because I would have expected noradrenalin to have a disinhibitory effect on the local circuit and therefore to act via VIP-CR interneurons – which does not seem to be the case. CCK-positive VIP cells as well as CCK-negative CR-negative VIP neurons (the fourth class found by the authors) were, however, activated by noradrenaline. So maybe all the disinibitory action through noradrenaline goes through CCK-negative CR-negative neurons? This definitely needs more follow-up experiments. I would be very curious to see these same experiments done in hippocampus …
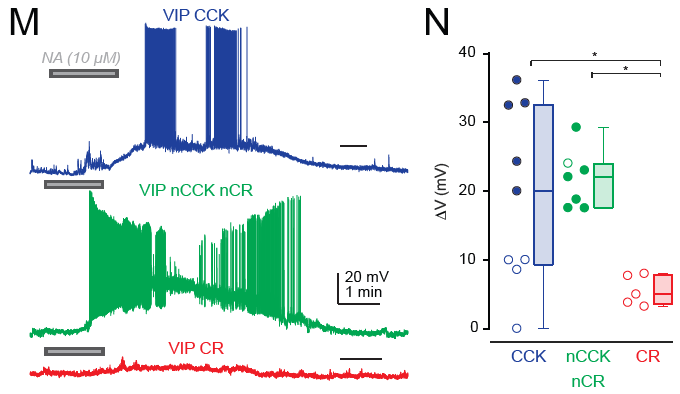
The main take-away from this paper is quite clear: perturbations of VIP neurons using transgenic VIP lines do affect multiple subtypes with partially opposing signature, with both disinhibitory effects (via CCK-negative interneurons) and inhibitory effects directly on pyramidal cells (via CCK-positive interneurons). This confound from using VIP transgenic lines is also discussed by the above study of Neubrandt, Lenkey et al., (2025). Targeting interneurons more specifically seems essential for future studies to manipulate or replicate the disinhibitory effect of (a subset of) VIP neurons, in neocortex as well as in hippocampus.
Hippocampal interneurons with all-optical methods
In an beautiful technical achievement, Yang et al. (2025) from Yoav Adam’s lab provide a systematic study of the functional characteristics of different neuron types in hippocampus (principal cells, VIP, SST, PV). They combine 1P voltage imaging (with the voltage sensors somArchon1 and QuasAr6b) and optogenetic activation of the same neurons (somCheRiff) to both record and manipulate specific neurons in vivo. Pretty cool! I liked how they focused on recording from and perturbing only very few neurons at a time, but at a apparently high quality.
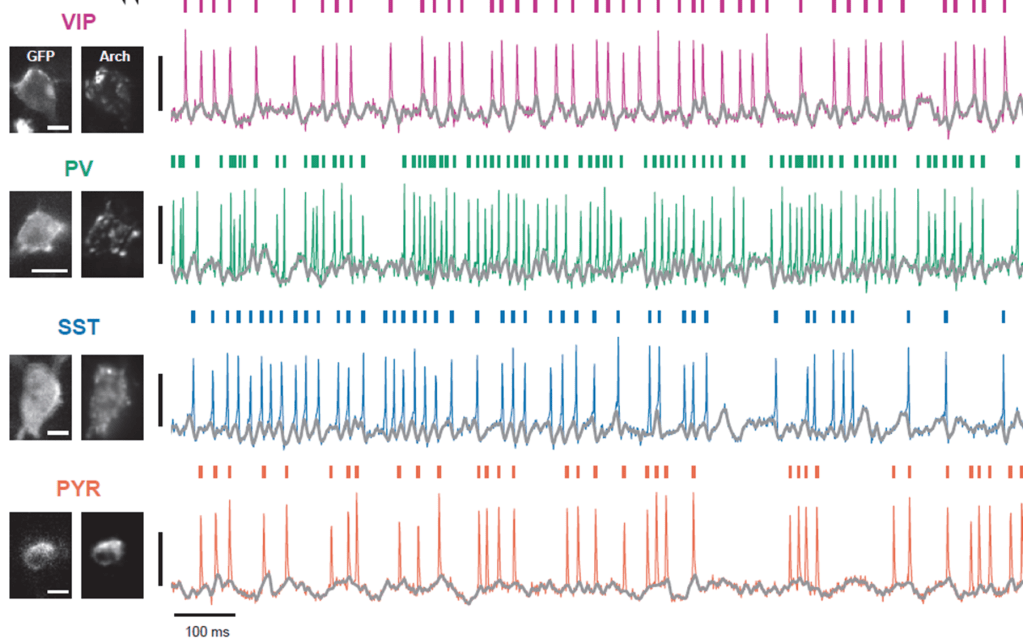
To describe these results, the authors analyze how optogenetic depolarization as well as state (walking vs. quiet) influences several cellular characteristics: the response to input, the amplitude of theta oscillations, and – for pyramidal neurons – the bursting propensity.
In a second step, Yang et al. use modeling to better interpret these descriptive findings. These single-cell conductance-based models are interesting to consider and consistent with the experimental results. However, I believe that the data itself and its pure description remain the strongest part of the paper. Such a dataset with voltage recordings and perturbations of several cell types in hippocampus would have been difficult to imagine just 10 or 15 years ago. I sincerely hope that the dataset will be curated and shared openly during the publication process!
.
References
Acsády, L., Görcs, T.J., Freund, T.F., 1996. Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience 73, 317–334. https://doi.org/10.1016/0306-4522(95)00609-5
Dellal, S., Zurita, H., Kruglikov, I., Valero, M., Abad-Perez, P., Geron, E., Meng, J.H., Prönneke, A., Hanson, J.L., Mir, E., Ongaro, M., Wang, X.-J., Buzsáki, G., Machold, R., Rudy, B., 2025. Inhibitory and disinhibitory VIP IN-mediated circuits in neocortex. https://doi.org/10.1101/2025.02.26.640383
Geiller, T., Vancura, B., Terada, S., Troullinou, E., Chavlis, S., Tsagkatakis, G., Tsakalides, P., Ócsai, K., Poirazi, P., Rózsa, B.J., Losonczy, A., 2020. Large-Scale 3D Two-Photon Imaging of Molecularly Identified CA1 Interneuron Dynamics in Behaving Mice. Neuron 108, 968-983.e9. https://doi.org/10.1016/j.neuron.2020.09.013
Gulyás, A.I., Hájos, N., Freund, T.F., 1996. Interneurons Containing Calretinin Are Specialized to Control Other Interneurons in the Rat Hippocampus. J. Neurosci. 16, 3397–3411. https://doi.org/10.1523/JNEUROSCI.16-10-03397.1996
Neubrandt, M., Lenkey, N., Vervaeke, K., 2025. VIP interneurons control hippocampal place cell remapping through transient disinhibition in novel environments. https://doi.org/10.1101/2025.02.01.636072
Turi, G.F., Li, W.-K., Chavlis, S., Pandi, I., O’Hare, J., Priestley, J.B., Grosmark, A.D., Liao, Z., Ladow, M., Zhang, J.F., Zemelman, B.V., Poirazi, P., Losonczy, A., 2019. Vasoactive Intestinal Polypeptide-Expressing Interneurons in the Hippocampus Support Goal-Oriented Spatial Learning. Neuron 101, 1150-1165.e8. https://doi.org/10.1016/j.neuron.2019.01.009
Yang, Q., Baror-Sebban, S., Kipper, R., London, M., Adam, Y., 2025. All-optical electrophysiology reveals behavior-dependent dynamics of excitation and inhibition in the hippocampus. https://doi.org/10.1101/2025.03.20.644347