During the last years, I have been working to understand not only neurons but also astrocytes and their role in the brain. The mode of action of astrocytes is dominated by a diversity of potential molecules and pathways involved and an almost equal diversity of opinions about what is the most important pathway. It is however clear that astrocytes sense many input molecules; there is a consensus that calcium might be a key player for intracellular signaling in astrocytes; and there are quite opposing views about the most relevant output pathways of astrocytes. In the following, I will discuss four recent papers on how astrocytes interact with neurons (and with blood vessels).
Norepinephrine Signals Through Astrocytes To Modulate Synapses
Do neuromodulators like noradrenaline act directly upon neurons, or are these effects mediated by, for example, astrocytes? In reality, it is not black or white, but an increasing number of scientists have acknowledged the potential big role played by astrocytes as intermediates (see e.g. Murphy-Royal et al. (2023)). In this study, Lefton et al. (2024) from the Papouin lab use slice physiology to carefully dissect such a signaling pathway from neuromodulators to astrocytes to neurons.
It is rare to see such consistent and convincing evidence for a complex neuromodulation signaling pathway as it is presented in a paper. To drive home the main messages, the authors apply many controls and redundant approaches from pharmacology, optogenetics. They use three different tools for astrocyte silencing (iBarks, CaleX and thapsigargin), conditional and region-specific knockouts and two-photon imaging to confirm their ideas. I think the paper is definitely worth the read. The main conclusion is that noradrenaline release in hippocampus silences pre-synapses of the CA3 -> CA1 pathway (the so-called Schaffer collaterals). This presynaptic effect is convincingly shown with several lines of evidence. The demonstrated mode of action of this pathway is the following: noradrenaline binds to alpha1-receptors of hippocampal astrocytes. Those astrocytes release ATP, which is metabolized to adenosine. Adenosine in turn binds to the adenosine A1-receptor that has been shown to locate at the CA3 -> CA1 presynapses, finally resulting in silencing of these synapses. Together, this cascade results in long-lasting synaptic depression on the timescale of minutes. Quite impressive work!
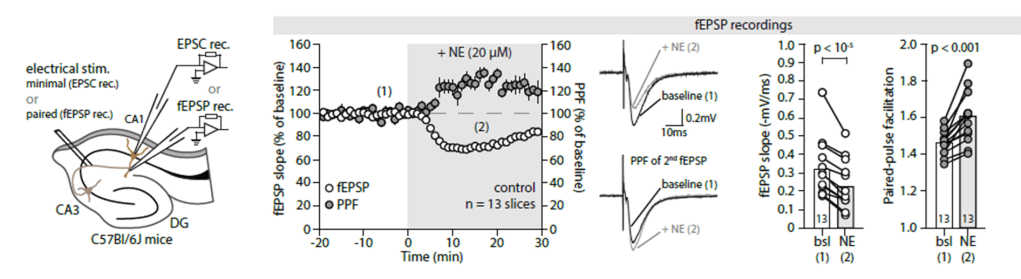
There are a few caveats to consider when interpreting the study. First, most of the work was done with a noradrenline concentration of 20 uM in the bath. This is relatively high, especially given previous work that showed somewhat opposite effects for sub-uM concentrations (Bacon et al., 2020). One can speculate that the physiological effect of the pathway found by Lefton et al. may therefore be weaker and, instead of fully silencing the presynaptic effects, rather tone down their relative importance compared to other inputs. The observed effect and signaling cascade is, however, interesting by itself.
Second, Lefton et al. convincingly show that the presynapses are depressed after noradrenaline release. This finding is also accurately reflected in the title. However, in some places, the finding is reframed as an “update of weights” in a non-Hebbian fashion, and “reshaping of connectivity”. This description is not wrong, but a bit misleading because these terms suggest an important role for memory and long-term potentiation, which is not how I would interpret the results. But this is just a minor detail.
Thinking about these results, I’m wondering how specific the effect is on the investigated CA3 -> CA1 synapses. It is an appealing idea to think that, e.g., synapses from entorhinal cortex (EC) onto CA1 might be less affected by this signaling pathway. This way, noradrenaline could be used to specifically reduce inputs from CA3 vs. inputs from EC. An obvious next step for a follow-up study would be to investigate the distribution of A1 receptors on different synapses, and the effect of noradrenaline via astrocytes on other projections to CA1.
Altogether, despite the caveats, this is really a nice paper, and it clearly shows the raw power of slice work when it is performed systematically and thoroughly. This work is particularly interesting as a companion paper describes a very similar pathway with noradrenaline, astrocytes and adenosine to silence not only neurons but also behavior (Chen et al., 2024).
A spatial threshold for calcium surge
Our own work has recently shown that astrocytic somata conditionally integrate calcium signals from their distal processes, and we have shown that the noradrenergic system is sufficient to trigger such a somatic integration (Rupprecht et al., 2024). In this conceptually related paper, Lines et al. (2023) from the Araque lab similarly describe conditional somatic activation of astrocytes, which they term somatic “calcium surges”. However, they use distal calcium signals rather than noradrenaline levels to explain whether these somatic calcium surges do occur or not.
Their main finding is a “spatial threshold”, i.e., a threshold of a minimum fraction of distal astrocytic processes that need to be activated in order to lead to somatic calcium surges. This is an interesting finding which they validate both in vivo and in slices in somatosensory cortex. The authors quantify that activation of >23% of the arborization results in a somatic calcium surge. Although I like the attempt to be quantitative and makes the results easier to compare to other conditions, I believe that the precise value of this threshold is a bit over-emphasized in the paper. I believe that this specific value could change quite a bit with different imaging conditions, with different analysis tools, or when assessing the calcium signals volumetrically in 3D instead of in a 2D imaging plane. However, I still like the overall approach, and I think it is quite complimentary to our approach focusing on noradrenaline as the key factor to control somatic integration. In the end, these two processes – noradrenaline signaling and activation of processes – are not mutually exclusive, but two processes are not only correlated with each other but that are also very likely to causally affect each other.
Figure 6 of the paper makes an additional step by establishing a connection between somatic calcium surges and gliotransmission and subsequent slow-inward currents in neurons. This connection is potentially of very big interest; however, I don’t think that the authors do themselves a favor by addressing this question in a short single figure at the end of an otherwise solid paper. But other readers might have a different perspective on that. In any case, I can only recommend checking out this interesting study!
How GABA and glutamate activate astrocytes
It is well-known that activation of neuronal glutamatergic or GABAergic synapses also activates astrocytes. Cahill et al. (2024) from the Poskanzer lab investigated this relationship systematically in slices using localized uncaging of glutamate and GABA. In particular the application or uncaging of glutamate lead to quite strong activation of astrocytic processes and somata. Very interesting experiments. The authors find that events locally evoked by GABA or Glu release propagate within – and across – astrocytes. This finding is, at least for me, quite unexpected, and I hope that it will be confirmed in future studies.
In addition, I believe that these experiments and results would be really useful to better understand somatic activation of astrocytes. Does simple stimulation with glutamate also result in somatic activation (in the spirit of “centripetal propagation” or “somatic calcium surges”), as one would expect from the analysis of Lines et al. (2023); or would it require the additional input from noradrenaline, as our results (Rupprecht et al., 2024) seem to suggest? A – in my opinion – interesting question that could be addressed with this dataset.
Astrocytic calcium and blood vessel dilations
It is well-known that astrocytes and in particular their end-feet interact with blood vessels. However, there has been a longstanding debate about the nature of these interactions. A big confound is that the observables (blood vessel dilations and astrocytic endfeet activation) might be connected via correlative rather than causal processes. For example, both actions might take place upon noradrenaline release but could be triggered independently by two separate signaling pathways without interaction directly.
In this fascinating paper, Lind and Volterra (2024) try to disentangle these processes by looking specifically at moments when the observed animals do not move. In this “rest” state, all these processes are less correlated with each other, enabling a better understanding of the natural sequence of events. In brief, the authors find that calcium signals in astrocytic endfeet seem to control whether a vessel dilation spreads across compartments or not. These analyses were enabled by imaging blood vessel dilation and astrocytic endfeet calcium in a 3D volume using two-photon microscopy in behaving mice. Great work!
Acknowledgements
The section on the paper by Lefton et al. (2024) is based on discussions with Sian Duss.
References
Bacon, T.J., Pickering, A.E., Mellor, J.R., 2020. Noradrenaline Release from Locus Coeruleus Terminals in the Hippocampus Enhances Excitation-Spike Coupling in CA1 Pyramidal Neurons Via β-Adrenoceptors. Cereb. Cortex 30, 6135–6151. https://doi.org/10.1093/cercor/bhaa159
Cahill, M.K., Collard, M., Tse, V., Reitman, M.E., Etchenique, R., Kirst, C., Poskanzer, K.E., 2024. Network-level encoding of local neurotransmitters in cortical astrocytes. Nature 629, 146–153. https://doi.org/10.1038/s41586-024-07311-5
Chen, A.B., Duque, M., Wang, V.M., Dhanasekar, M., Mi, X., Rymbek, A., Tocquer, L., Narayan, S., Prober, D., Yu, G., Wyart, C., Engert, F., Ahrens, M.B., 2024. Norepinephrine changes behavioral state via astroglial purinergic signaling. https://doi.org/10.1101/2024.05.23.595576
Lefton, K.B., Wu, Y., Yen, A., Okuda, T., Zhang, Y., Dai, Y., Walsh, S., Manno, R., Dougherty, J.D., Samineni, V.K., Simpson, P.C., Papouin, T., 2024. Norepinephrine Signals Through Astrocytes To Modulate Synapses. https://doi.org/10.1101/2024.05.21.595135
Lind, B.L., Volterra, A., 2024. Fast 3D imaging in the auditory cortex of awake mice reveals that astrocytes control neurovascular coupling responses locally at arteriole-capillary junctions. https://doi.org/10.1101/2024.06.28.601145
Lines, J., Baraibar, A., Nanclares, C., Martín, E.D., Aguilar, J., Kofuji, P., Navarrete, M., Araque, A., 2023. A spatial threshold for astrocyte calcium surge. https://doi.org/10.1101/2023.07.18.549563
Murphy-Royal, C., Ching, S., Papouin, T., 2023. A conceptual framework for astrocyte function. Nat. Neurosci. 26, 1848–1856. https://doi.org/10.1038/s41593-023-01448-8
Rupprecht, P., Duss, S.N., Becker, D., Lewis, C.M., Bohacek, J., Helmchen, F., 2024. Centripetal integration of past events in hippocampal astrocytes regulated by locus coeruleus. Nat. Neurosci. 27, 927–939. https://doi.org/10.1038/s41593-024-01612-8
Pingback: How to use Google Scholar as a neuroscientist | A blog about neurophysiology